
Describe and compare the nature and arrangement of chemical bonds in ionic compounds, simple molecules, giant covalent structures, and polymers and metals.4.6 Interactions over small and large distances.Students should be able to explain chemical bonding in terms of electrostatic forces and the transfer or sharing of electrons.Ionic bonding occurs in compounds formed from metals combined with non-metals.For ionic bonding the particles are oppositely charged ions.The ions produced by metals in Groups 1 and 2 and by non-metals in Groups 6 and 7 have the electronic structure of a noble gas (Group 0).Non-metal atoms gain electrons to become negatively charged ions.Metal atoms lose electrons to become positively charged ions.When a metal atom reacts with a non-metal atom electrons in the outer shell of the metal atom are transferred.4.2.1 Chemical bonds, ionic, covalent and metallic.
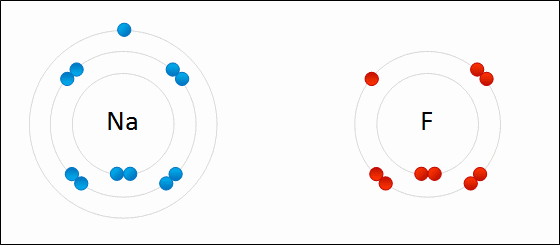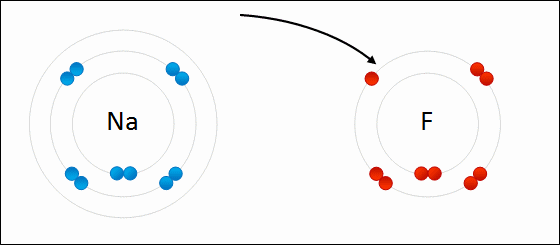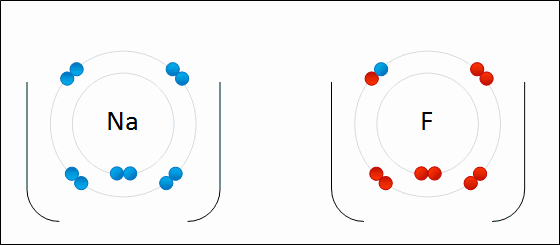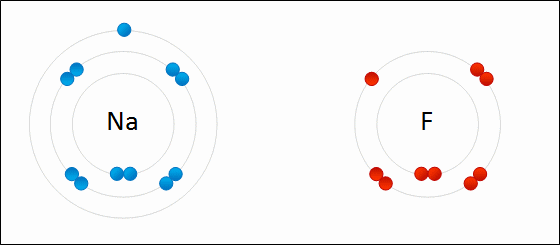 4.2 Bonding, structure and the properties of matter. know that ionic bonding is the strong electrostatic attraction between oppositely charged ions Ionic bonding involves electrostatic attraction between oppositely charged ions in a lattice. a) ionic bonding as electrostatic attraction between positive and negative ions, and the construction of 'dot-and-cross' diagrams. Examination of a model of the NaCl crystal. Representation of ionic bonds using dot and cross diagrams. 1.2.4 demonstrate knowledge and understanding that: ionic bonding involves attraction between oppositely charged ions ionic bonds are strong and substantial energy is required to break ionic bonds. 1.2.2 and 1.2.3 define the terms cation and anion and explain, using dot and cross diagrams, how ions are formed and how ionic bonding takes place in simple ionic compounds, limited to elements in Groups 1 (I) and 2 (II) with elements in Groups 6 (VI) …. Unit C1: Structures, Trends, Chemical Reactions, Quantitative Chemistry and Analysis. 1.2.3 demonstrate knowledge and understanding that: ionic bonding involves attraction between oppositely charged ions ionic bonds are strong and substantial energy is required to break ionic bonds. 1.2.2 define the terms cation and anion and explain, using dot and cross diagrams, how ions are formed and how ionic bonding takes place in simple ionic compounds, limited to elements in Groups 1 (I) and 2 (II) with elements in Groups 6 (VI) and 7 (VII),…. Unit 1: Structures, Trends, Chemical Reactions, Quantitative Chemistry and Analysis. 1.3.1 demonstrate understanding that ionic bonding is the electrostatic attraction between oppositely charged ions formed by electron transfer.
4.2 Bonding, structure and the properties of matter. know that ionic bonding is the strong electrostatic attraction between oppositely charged ions Ionic bonding involves electrostatic attraction between oppositely charged ions in a lattice. a) ionic bonding as electrostatic attraction between positive and negative ions, and the construction of 'dot-and-cross' diagrams. Examination of a model of the NaCl crystal. Representation of ionic bonds using dot and cross diagrams. 1.2.4 demonstrate knowledge and understanding that: ionic bonding involves attraction between oppositely charged ions ionic bonds are strong and substantial energy is required to break ionic bonds. 1.2.2 and 1.2.3 define the terms cation and anion and explain, using dot and cross diagrams, how ions are formed and how ionic bonding takes place in simple ionic compounds, limited to elements in Groups 1 (I) and 2 (II) with elements in Groups 6 (VI) …. Unit C1: Structures, Trends, Chemical Reactions, Quantitative Chemistry and Analysis. 1.2.3 demonstrate knowledge and understanding that: ionic bonding involves attraction between oppositely charged ions ionic bonds are strong and substantial energy is required to break ionic bonds. 1.2.2 define the terms cation and anion and explain, using dot and cross diagrams, how ions are formed and how ionic bonding takes place in simple ionic compounds, limited to elements in Groups 1 (I) and 2 (II) with elements in Groups 6 (VI) and 7 (VII),…. Unit 1: Structures, Trends, Chemical Reactions, Quantitative Chemistry and Analysis. 1.3.1 demonstrate understanding that ionic bonding is the electrostatic attraction between oppositely charged ions formed by electron transfer. 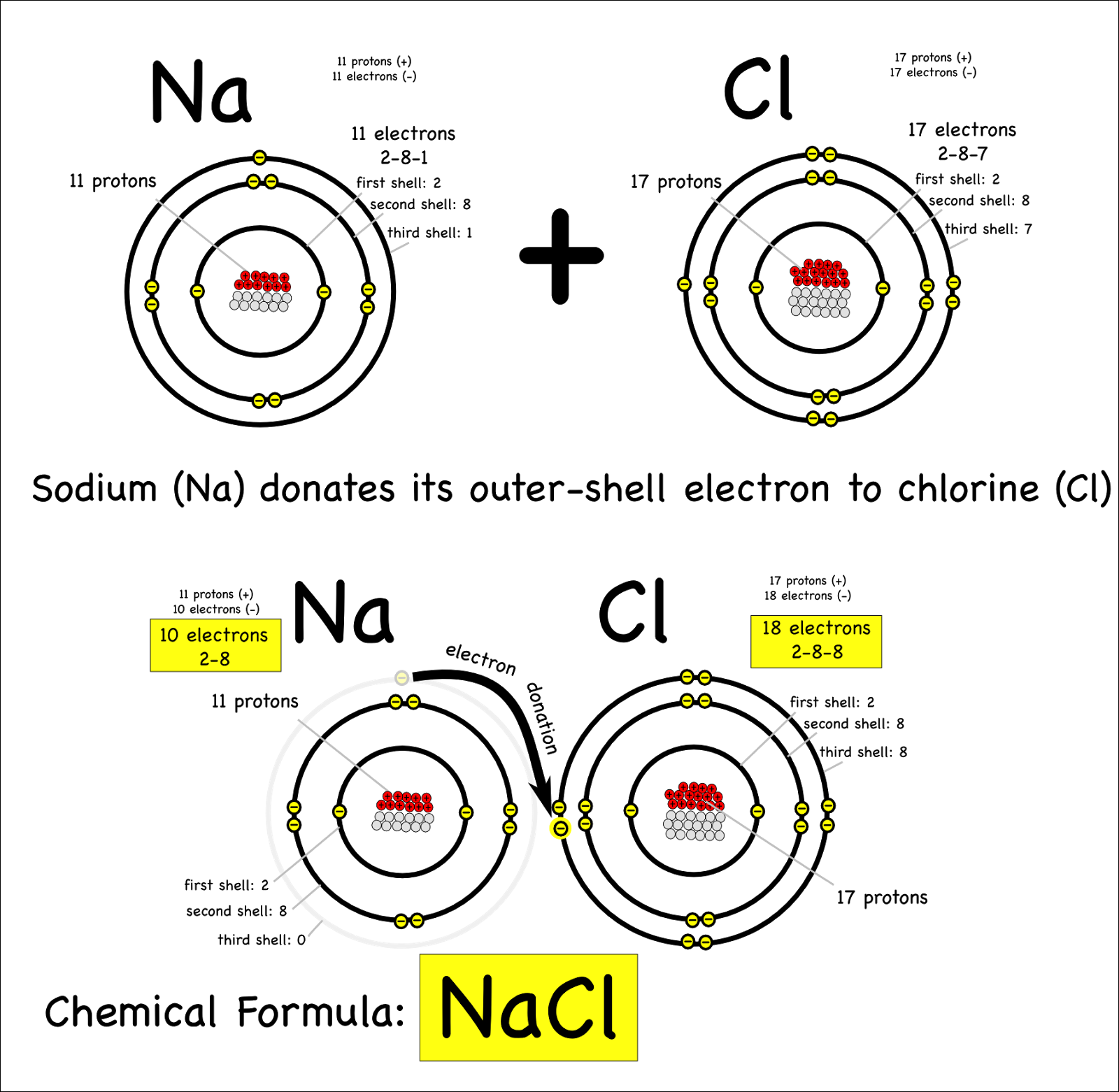
Unit AS 1: Basic Concepts in Physical and Inorganic Chemistry.(a) ionic bonding in terms of ion formation and the interaction between positive and negative ions in an ionic crystal.Unit 1: THE LANGUAGE OF CHEMISTRY, STRUCTURE OF MATTER AND SIMPLE REACTIONS.Hamied Inspirational Chemistry Programme.Introductory maths for higher education.The physics of restoration and conservation.






 0 kommentar(er)
0 kommentar(er)
